

In fact, it's a sign you're eating corn in one of its healthiest forms. Corn kernels in your poo might be odd, but they're not bad for your health.
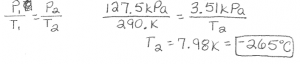
A look at the nutrition facts show common processed corn products, like corn oil and high fructose corn syrup, lose most of the beneficial fiber and nutrients during processing. The vast majority of that corn is not the hard to digest kernels nibbled off the cob, but corn that's been turned into soft tortillas, chips, popcorn and - the big one - high fructose corn syrup.Įasier to digest, however, is not to be confused with healthier. The Tufts University report estimates that each American consumes 160 lbs. In fact, the majority of the corn you eat is processed. Grinding, wet milling, cooking - every processing step breaks down those hard to digest fiber molecules a little further, she said. That's true for humans and animals alike. "The more you process it, the easier it is to digest," Watson said. There's a way to make corn more digestible and disappear from your poop altogether: processing. What's the difference between a fruit and a vegetable? Why do some fruits and vegetables conduct electricity? For instance, if you need 1 meat and two bread in order to make one sandwich and you had 100 bread but only 5 meat, then you can only make 5 sandwiches even though you will have a lot of bread left over.- Why are bananas berries, but strawberries aren't? This idea can be applied to making sandwiches. When 12.0 g of iron rusts?4Fe(s) + 3O2(g) → 2Fe2O3(s)Ħ. What real-life applications can this concept of limiting and excess reagents be applied to? Because the reactants were small the products where also small.ĥ. Rust is produced when iron reacts with oxygen. The smallest balloon was blue because it had the least amount of baking soda go into it (1 g). The reactants where large so the products where as well.Ĥ. Which balloon was the smallest? Explain. With the vinegar this caused the largest reaction. The green balloon was the largest because that was the flask we put the most baking soda into (4 g). Because of this when there is less reactants the out come will be less as well.ģ. Which balloon was the largest? Explain. How is the amount of product in a reaction affected by an insufficient quantity of any of theĪccording to the law of conservation of mass, there will always be the same amount matter on the reactants side as the products side. We were able to conclude this by noticing that there was vinegar left over but all of the baking soda had been used up in the reaction.Ģ. The excess reagent was the baking soda and the excess reagent was the vinegar.
#CHEMLAB 13 DETERMINE PRESSURE IN POPCORN KERNELS ANSWERS CRACK#
This would allow the rocket to shoot up by itself without outside assistance, hopefully improving the height reached. 1) They don't have enough water in them to generate enough pressure 2) There's a crack in the kernel which lets out heat during process, so no pressureno explosion 3) There's not enough heat to pressurize the kernel 4) There's an unequal distribution of heat or oil Predict what the relationship between pressure and temperature is. For next time, I think it would have been better to tighten the cork just enough but to much like we did in our first attempt. When it comes to gas laws, my group believes boyels law was in effect, because the P1 and V2 were represented by the coke before the montos, and then after the new P2 and V2 were discovered. When he does this the angle of trajectory goes from vertical to horizontal and we are unable to really tell how high our rocket could have launched. As you can see in the video below, we sealed the bottle so tight that it couldn't burst without Mr. Our rocket did not have such a glorious launch. At least this is how it is supposed to work. Because of the cork sealing off the exit for the CO2, the pressure builds up in the bottle until it bursts through cause the rocket to take flight. When the mentos go into the Coke, they make the coke fizz excessively with CO2 because the Coke is carbonated in the first place. I call it this because during the reaction the coke remains coke and the mentos stay mentos. The reason a rocket like this would launch would be because of a physical reaction. Wong's 6th period class attempted to use our knowledge of gas laws to launch Coke and mentos rockets.


 0 kommentar(er)
0 kommentar(er)
